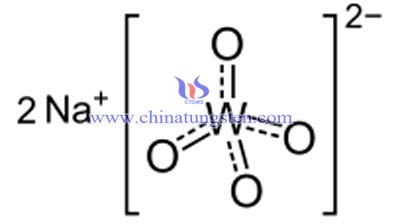
Sodium tungstate, Na2WO4, a tungstate of sodium, is useful as a source of tungsten. It is prepared from tungsten ores used to manufacture tungsten by reducing it. It is often found as the dihydrate, Na2WO4•2H2O. This salt is soluble in water and is a moderately strong oxidizing agent. Like the molybdate, the deep-coloured complex tungstate(VI,V) is formed on reducing the compound with a very mild reducing agent, such as complex organic compounds.
Variants of sodium tungstate:
sodium tungstate or sodium wolframate
sodium tungstate or sodium wolframate
Properties: colorless crystal or white crystalline powder. In dry air weathering, lose crystal water at 100 ℃. Soluble in water, soluble in ethanol. The relative density of 3.23 ~ 3.25. Melting point 698 ℃ (dry goods).